
Unfortunately, that source isn't readily viewable online (all I found was what appears to be a German version, at Berichte der Bunsengesellschaft für physikalische Chemie 1990, 94 (1), 93–93 (It's behind a paywall.) But if you could get your hands on it (interlibrary loan, say), it should contain a detailed description of how the values were actually calculated.įWIW, here's a link showing how standard molar entropies might be calculated (but I don't know if the procedure described here is what is used to determine the official CODATA values): Standard Molar Entropy of Solid Aluminum Oxide, Comments to Tandy Grubbs, Stetson University ( via the Internet Archive).Įssentially, it mentions using the Debye extrapolation I mentioned in an earlier comment up to $\approx\pu$, the fact that the system is locked into only one microstate (even if it's not a perfect crystal) gives it zero entropy. A., CODATA Key Values for Thermodynamics, Hemisphere Publishing Corp., New York, 1989. Here's a link to the official CODATA site showing the values:Ĭox, J. Not quite an answer, but too long for a comment: What is the precise definition (preferably with reference) of standard molar entropy?
#Standard molar entropy how to
Most of the following issues will be covered by the exact definition, but to be more elaborate, these immediately come to my mind: what exactly is the standard state for each element? Do all elements form a perfect crystal at 0 K, and how can we prove this? Should I think of the third law (zero entropy at zero kelvin) as being a definition that sets an otherwise arbitrary baseline, or can it be proven that the quantum mechanical von Neumann entropy really is zero for pure elements at 0 K?Īn internet search turned up plenty of information about the values of standard molar entropy for various compounds and how to use them, but I wasn't able to find a resource with a detailed description of its definition. However, I'm not even sure that's exactly correct, and there are a lot of details that need to be filled in if it is right. The standard transformed Gibbs energy of reaction provides the criterion for spontaneous change and equilibrium for an enzyme-catalyzed reaction at. Then, for the compound of interest, calculate and/or measure the entropy difference between the compound and its constituent elements at 0 K.
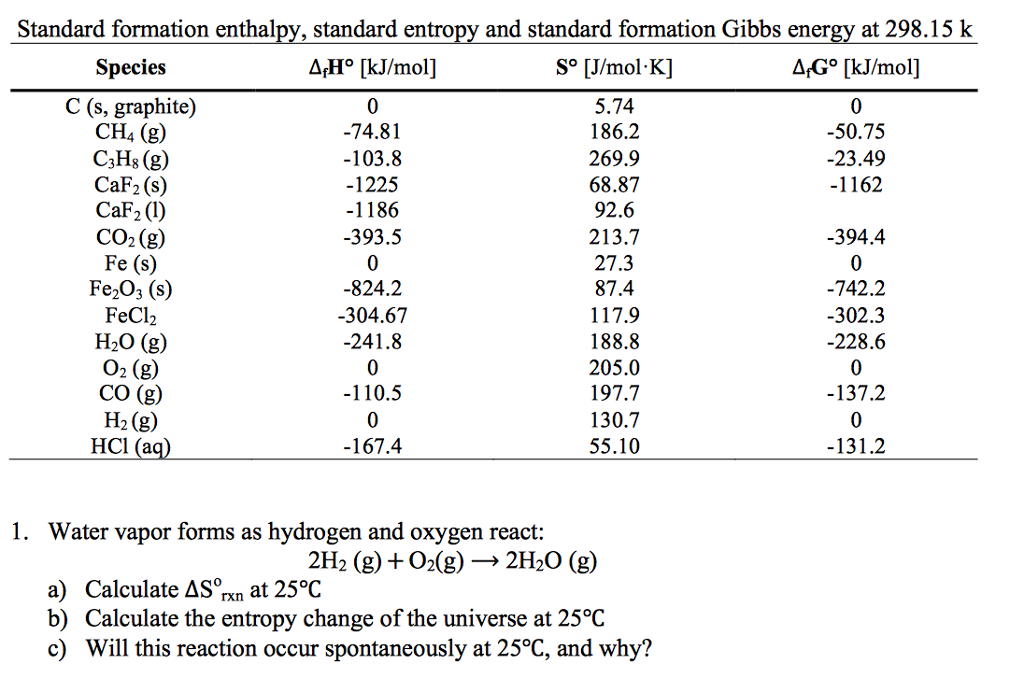


My recollection from a long time ago is that it goes roughly like this:įor each element, assume that its entropy goes to zero at 0 kelvin when it's in some standard state So, randomness will decrease and thus entropy will decreases.(As entropy is measurement of. The standard molar entropy is usually given the symbol S°, and has units of joules per mole kelvin (J mol 1 K 1).Unlike standard enthalpies of formation, the value of S° is an absolute. I understand what the standard molar entropy is, and how to use it in calculations, but I'm interested in understanding exactly how it's defined and measured. clearly, number of gas molecules decreased in the reaction. In chemistry, the standard molar entropy is the entropy content of one mole of substance, under standard conditions (not standard temperature and pressure STP).


 0 kommentar(er)
0 kommentar(er)
